Startup Beddr today launched the "SleepTuner," the first FDA-registered consumer sleep wearable that helps to assess and improve sleep quality. SleepTuner is a small accessory (about the size of a postage stamp) with Bluetooth Low Energy that attaches to your forehead to measure sleep duration, track heart rate, optimize nighttime breathing, and improve oxygenation, all viewable when synced to the compatible iOS app.

Specifically, SleepTuner was built to help accurately measure oxygen and breathing overnight in order to determine if the user suffers from sleep apnea, a disorder in which breathing repeatedly stops and starts during sleep. Beddr says that its tracker includes "the essentials of a traditional sleep lab" to measure blood oxygen levels, heart rate, sleep position, and stopped breathing events.
To use, SleepTuner is attached to the forehead by a hypoallergenic disposable adhesive, which the company says avoids the pitfalls of rival options that have numerous wires and other attachments. The device has a 50 mAh battery that provides up to 20 hours of continuous test time, and is rechargeable. SleepTuner has optical sensors and a 3-axis accelerometer to gather sleep data, which is saved in the Beddr Cloud until the user syncs it with the iOS app in the morning.
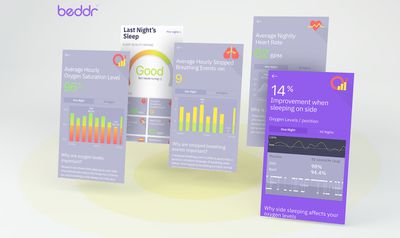
Once synced, the app gathers data from SleepTuner over the course of its use, providing users with helpful insights like determining their optimal sleeping position, overall sleep quality, pointing out how often they wake up, highlighting night-to-night improvements, and more.
Beddr compares SleepTuner to a few other sleep-tracking capable products on its website, including Apple Watch Series 4 and Beddit:
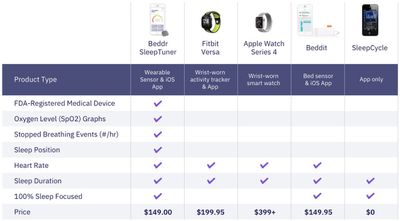
SleepTuner is available to pre-order on Beddr's website for $149, including the sensor, 12 adhesive strips, a charging cable, and a protective case. The device will begin shipping next month.



















Top Rated Comments
Seems like an early April Fools joke.
The amount of frankly ignorant and well, stupid posts for this article is off the charts.
Sleep apnea is a major killer. Most are undiagnosed.
This has an SpO2 sensor in it. It can detect pulse rate and most importantly, oxygenation levels. Yes, the forehead is a good site for such a sensor. Otherwise, the good sites are the earlobe and fingers, and there are issues with those sites while sleeping.
Medical tape is used all the time in... guess what - medical situations, and this is one of those.
Hypoallergenic because some people - like me - react to some types of tape adhesives.
But hey, I actually worked on and helped to design SpO2 devices when they first came out, so what do I know? And that work extended into sleep apnea studies... with a 5 pound box of gear and leads and what have you. You know, the stuff that actually impacted sleep patterns.
If this works (and I have a lot more questions on this, I have no connection to the vendor), it's damn near the ideal device for in-home sleep apnea testing.
Since this is a Bluetooth device... Why the heck is a person's sleep patterns uploaded to their server? Totally unnecessary.
Data mining at its finest. Why would anyone trust this obscure company with their most intimate health data?
The accelerometer will detect movement, should be able to do some detection of sleep levels.
Overall, if all the device does is confirm you have sleep apnea (or rule it out), it's a winner. Have it? Go get treatment (CPAP).
I'd need to see a lot more to understand what sort of recommendations it might be able to generate.